Sun Jialin
Nanyang Technological University, Singapore
Title: Structural and functional studies of actin interacting protein 5, a novel actin assembly regulator in Saccharomyces cerevisiae
Biography
Biography: Sun Jialin
Abstract
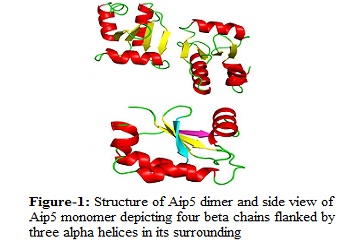
Aip5 is a newly identified member of the polarisome complex found in yeast. It acts as actin assembly regulator via binding to other protein partners in the polarisome complex. Polarisome complex helps to establish the polarity of the cell, which is critical for cell growth and division. It is also responsible for actin filament assembly in the cell that contributes to the tissue organization and cell motility. All in all, polarisome is crucial for the survival and division of a yeast cell. Being a new member of the polarisome complex, Aip5 is most likely recruited by its binding partner Spa2 to the tip of the yeast bud during polarized cell growth, to regulate actin filament assembly. Deletion of Spa2 led to the complete loss of localization signal of Aip5 to the polarized region in the cell, while deletion of Aip5 C-terminal had similar effect. These results indicate that Aip5, most probably interact with other polarisome proteins via its C-terminal. Thus it is critical to elucidate the structure of Aip5 given its importance in aiding the actin filament assembly. In this study, we have successfully solved the structure of Aip5 C-terminal at atomic resolution of 1.8 Å. It consists of a dimer per asymmetrical unit, which is consistent with the gel filtration chromatography result. This indicates that Aip5 possibly functions as a biological dimer as well. Preliminary results have shown that the oligomer state of Aip5 might play a part in actin filament assembly. More studies will be conducted to further investigate on the effect of Aip5 oligomer state with to its function