Yasuo Norikane
NIAIST, Japan
Title: Light-induced crawling motion of azobenzene crystals on a glass surface
Biography
Biography: Yasuo Norikane
Abstract
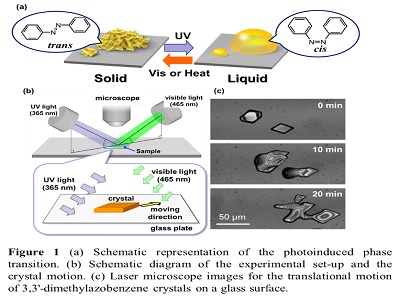
Creating self-propelled motion in nano- to macroscopic sized objects has been of interest to scientists. Physical or chemical energy has been used to obtain motion of liquid droplets or solid objects in various media. Using light as an energy source, photochromic molecules have been utilized to induce various mechanical motions of objects. In many cases, the mechanical motions involve the photochemical reactions of azobenzene molecules, which exhibit photoisomerization between trans and cis isomers. So far, various type of motion has been reported; however, photo-induced translational motion of crystals on solid surfaces is unknown. Recently, we have been interested in photochemically-induced phase transitions between solid and liquid phases in photochromic organic compounds such as macrocyclic or rod-shaped azobenzene derivatives. Materials’ showing the phase transition has been of interest because of its interesting properties such as photocontrollable adhesives, photoresists and motion on water surface. Here, we report the novel crawling motion of crystals of simple azobenzenes such as azobenzene and 3,3'- dimethylazobenzene on a glass surface. The motion is directional and continuous when irradiated simultaneously with two different wavelengths (365 and 465 nm). Our method is simple as crystals move on a bare glass surface using a light-emitting diodes or Hg lamp as light sources in a fixed position. The direction of the motion can be controlled by the position of the light sources and the crystals can even climb vertical surfaces. The motion proceeds without changing the crystal orientation, despite the large deformation of the crystal shape. There is a no need for any special treatment such as chemical modification, spatial gradient or application of ratchet potential of the solid surface. It is presumed that the motion is driven by crystallization and melting at the front and rear edges of the crystal, respectively, via photochemical conversion between the crystal and liquid phases induced by the trans-cis isomerization of azobenzenes